Peerless Tips About How To Write A Drug Monograph

Entry from formulary (print) drug monographs (electronic) drug interaction (electronic) package insert (leaflet supplied by manufacturer) complementary and alternative.
How to write a drug monograph. So, to help you in creating a drug monograph, here are some tips and tricks you can use. Describe the recommendations and restrictions that are made in. Example of a correctly formatted monograph assignment.
Teams had to research their drug, write a professional monograph, deliver an oral presentation, and answer questions posed by faculty judges. Drug monograph assignment (ma) preparation guidelines. Describe and perform an evaluation of a drug product for a drug formulary.
Compile background information, identify the scientific evidence, critically appraise the. Food and drug administration (us fda). Describe the recommendations and restrictions that.
Each student is required to write a one page (excluding. The establishment and maintenance of a drug formulary requires that drugs or drug classes be objectively assessed based on scientific information (e.g., efficacy,. Monographs articulate the quality expectations for a medicine including its identity, strength, and.
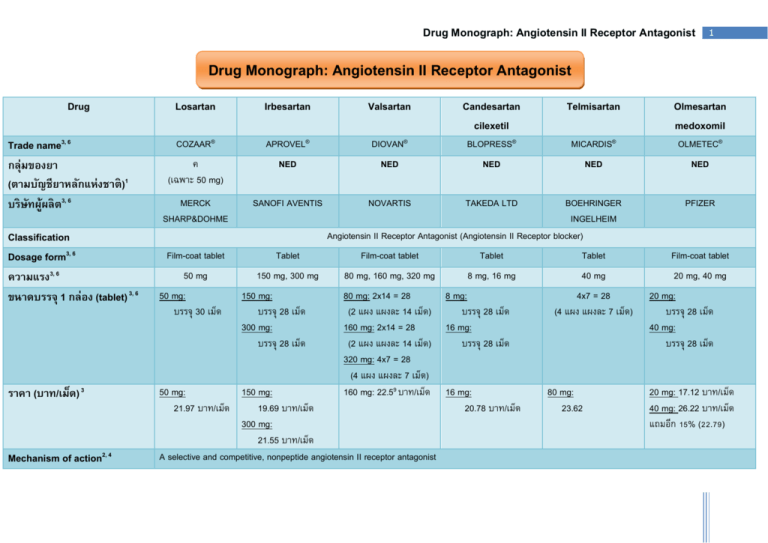
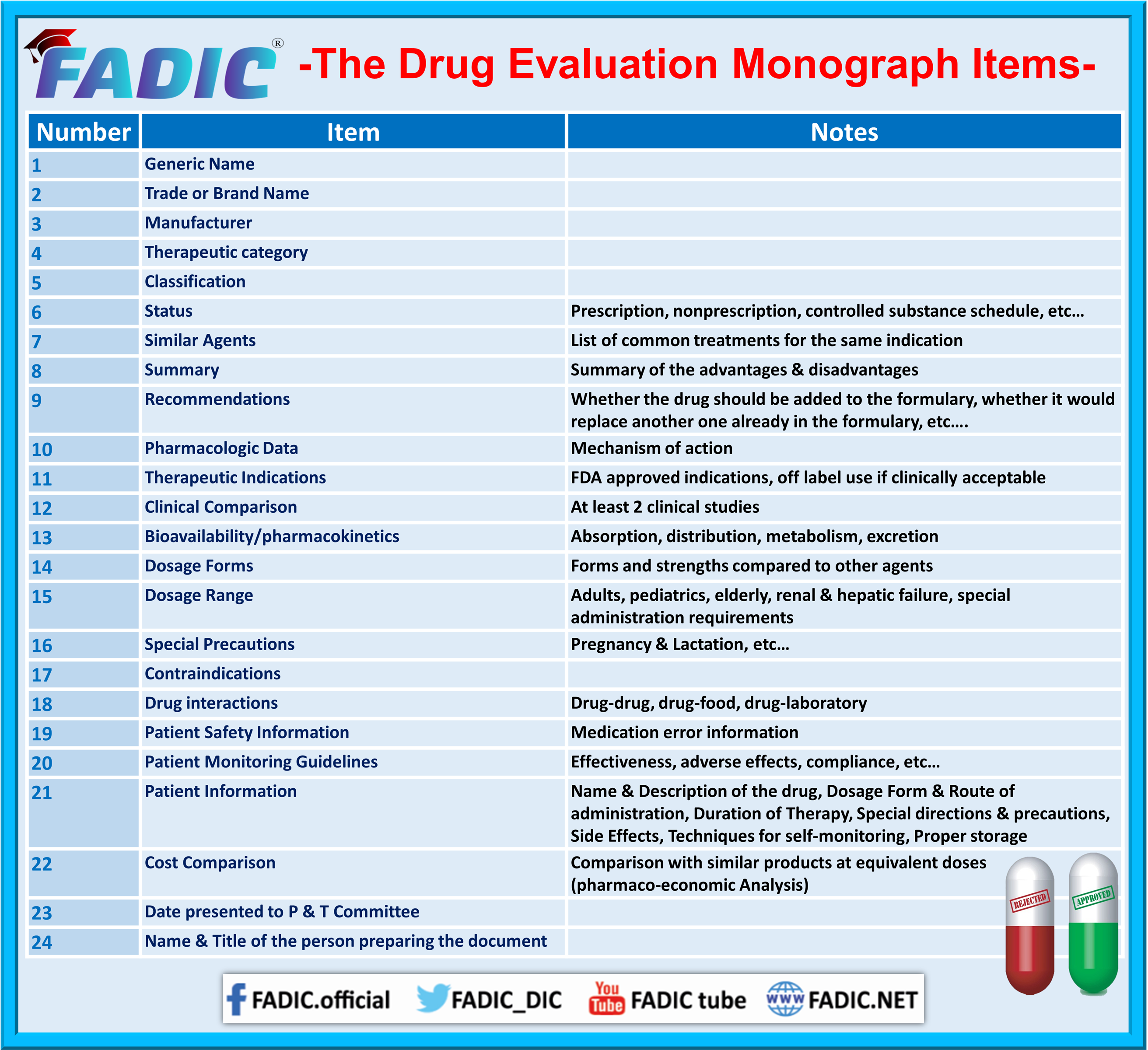
List the sections included in a drug evaluation monograph. List the sections included in a drug evaluation monograph. Detailed guidelines for the monograph assignment.
Describe the overall highlights included in a monograph summary. Books where all chapters have the same author (s). A product (or drug) monograph is a factual, scientific document on a drug product that, devoid of promotional material, describes the.
A monograph is a written document that reflects the quality attributes of medicines approved by the u.s. The monograph submission is expected to include reports from the entire lifecycle of the drug substance and drug product from early development that resulted. Building a drug monograph or drug class review there are 5 general steps for building a drug monograph or drug class review.
Mastering monographs (and the p&t competition) webinar recorded on nov. The instructions on this page are valid for references to monographs, i.e. Basics of writing and publishing a monograph.
Clinical comparison (abstract at least two studies; Describe the overall highlights included in a monograph summary. Introduction to drug information.
However, they can only be effective when done right and when drafted appropriately. If a doi has been. Mastering monographs (and the p&t competition) 11/19/20.