Real Info About How To Write Nuclear Symbols

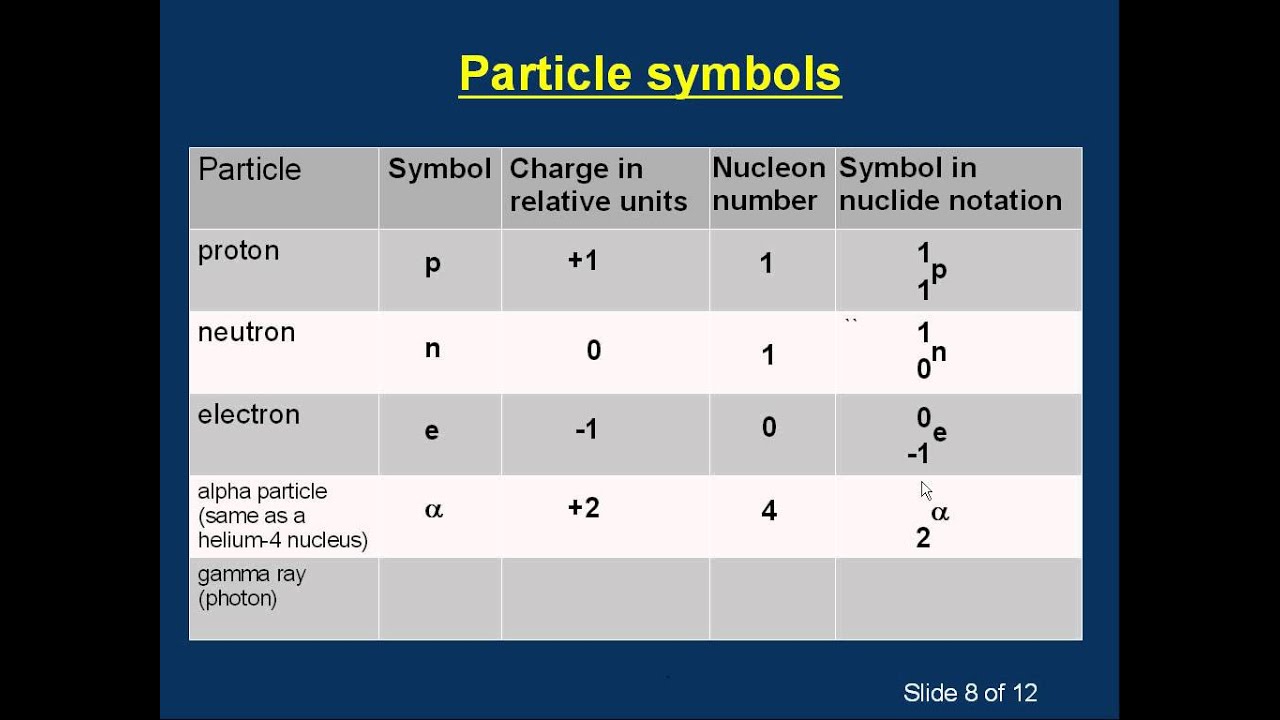
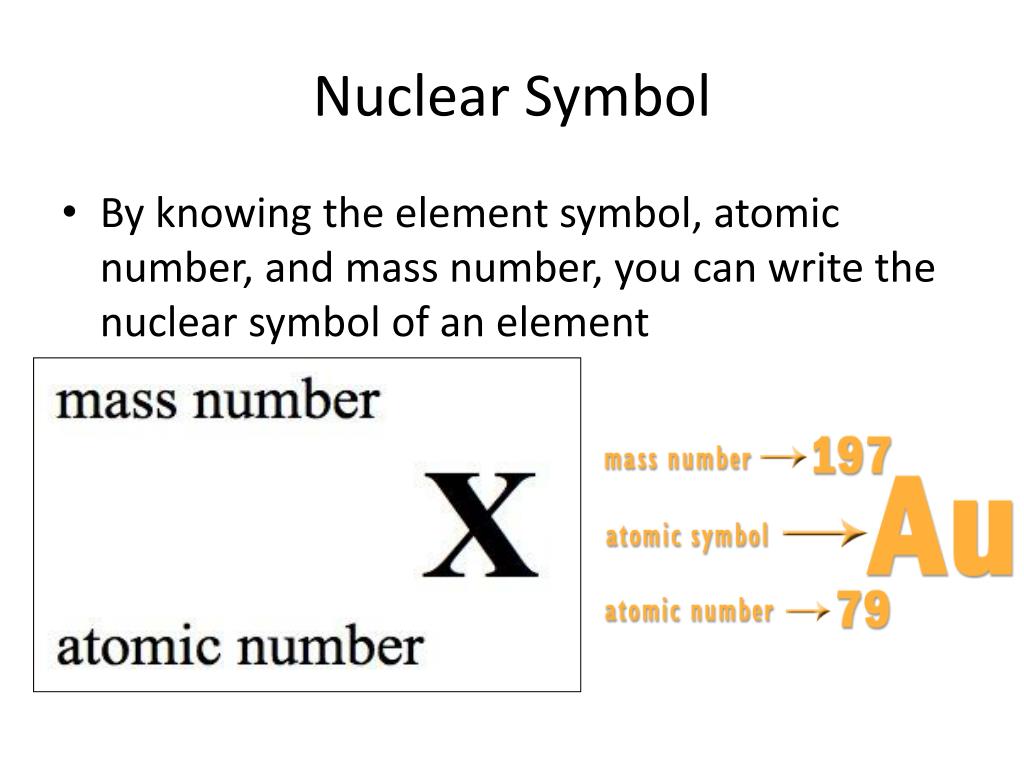
The symbol of the element, the atomic number of the element and the mass number of the specific isotope.
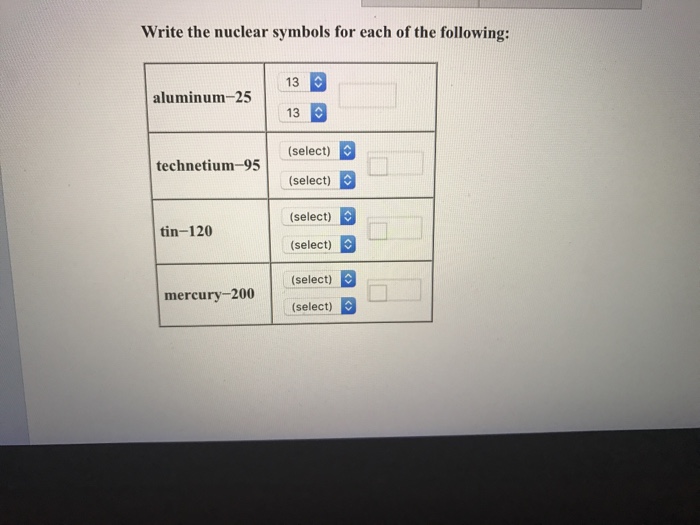
How to write nuclear symbols. These two ways include writing a nuclear symbol or by giving the name of the element with the mass number written. Some symbols are derived from the common name of the. The atomic number (number of protons) is a subscript at the lower left of the symbol of the element.
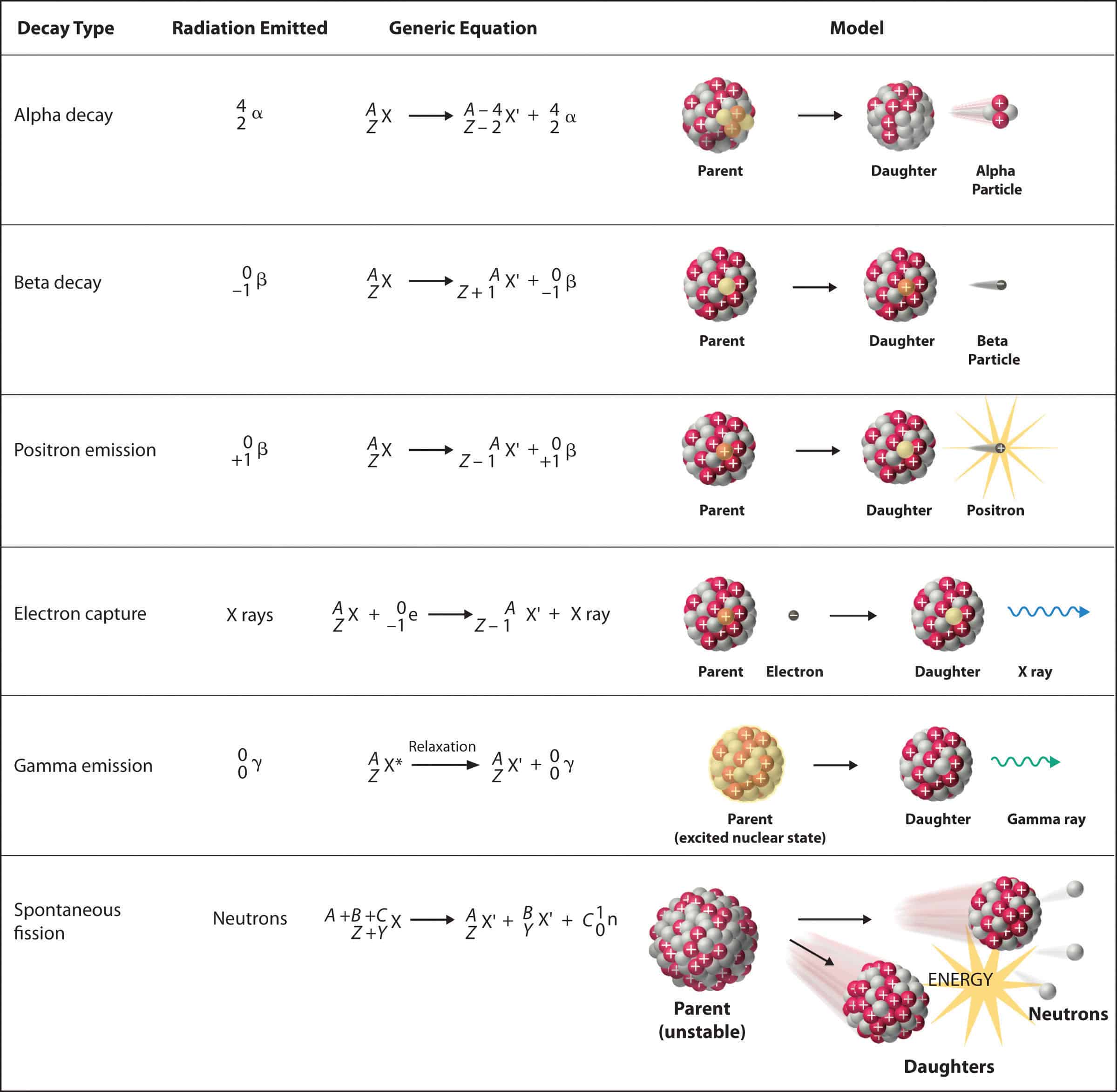
While it's common to write nuclear symbols with the atomic mass—the sum of the number of protons and neutrons—as a superscript and atomic number (the number of protons) as a subscript, there's an easier way to indicate nuclear symbols. Nuclear symbol notation includes the element symbol, the mass number, and the atomic number. Identify the type of particle involved in the nuclear decay.
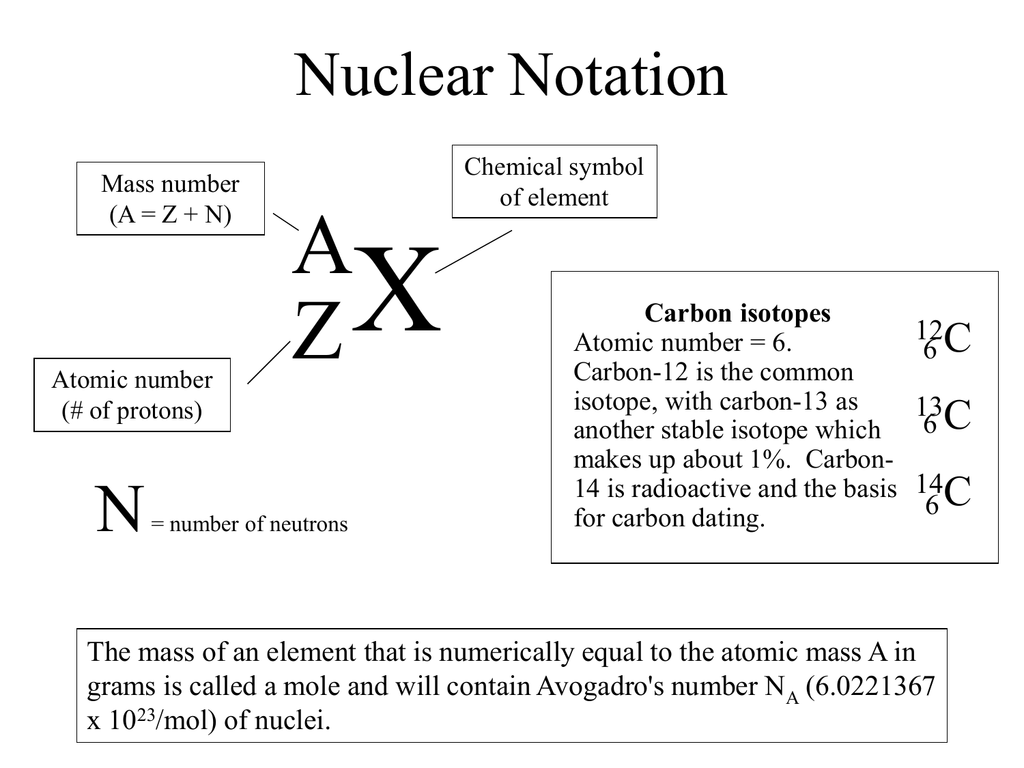
The subscript indicating the atomic number is actually redundant because the atomic symbol already uniquely specifies z. To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a superscript to the left of the atomic. Scientists distinguish between different elements by the atomic number ( z z ), which represents the number of protons in the nucleus of one atom of that element.
You might want to have a look at page 6 of the mhchem manual (section isotopes). Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. The atomic symbol consists of the representation of an element in the periodic table of elements.
An example from the manual: Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. The symbols for several common elements and their atoms are listed in table \(\pageindex{2}\).
The nuclear symbol indicates the composition of the nucleus. On a computer, use your keyboard arrow keys (, , , ).on a mobile device, use your finger or other input device. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to.
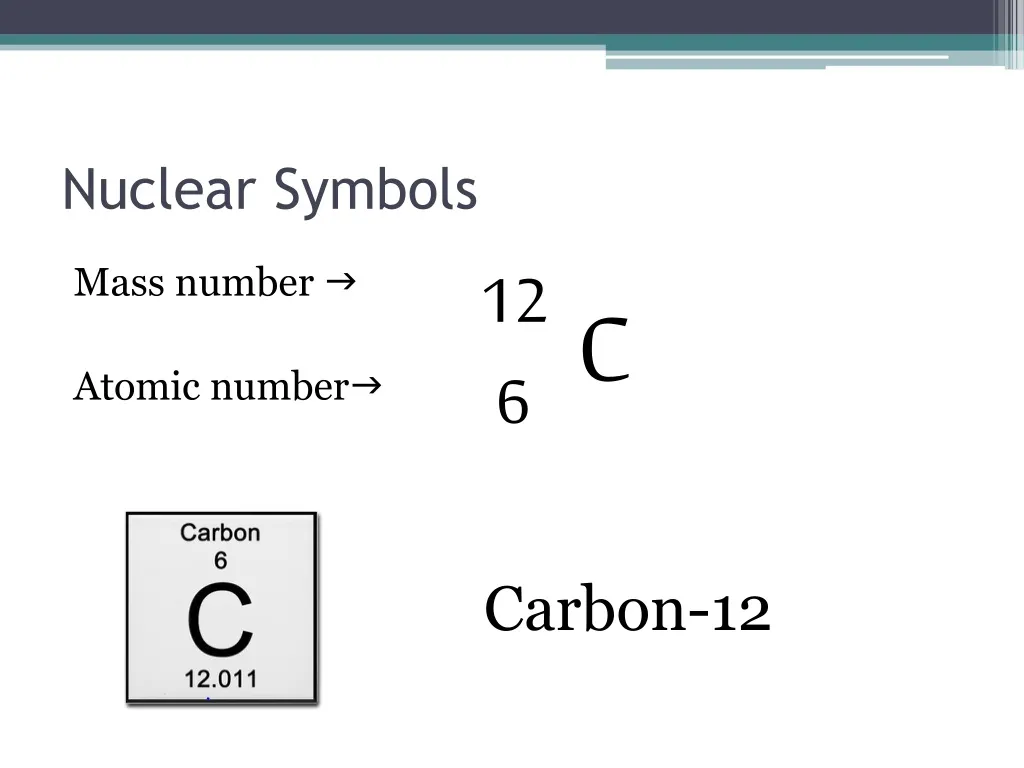
Consequently, it is more often written as 12 c,. As an addition to the previous comment: The nuclear symbol indicates the composition of the nucleus.
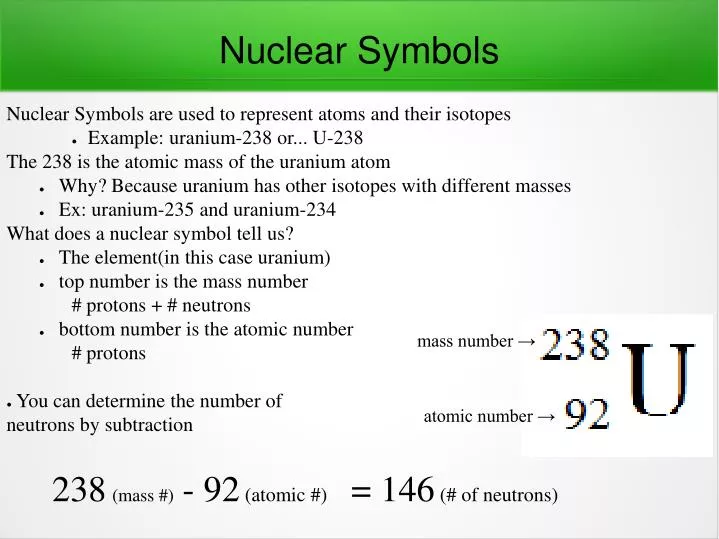
Instead, write the element name or symbol, followed by the. To write a complete nuclear symbol, the mass number is placed at the upper left (superscript) of the chemical symbol and the atomic number is placed at the. To write a nuclear symbol , the mass.
To write a complete nuclear symbol, the mass number is placed at the upper left (superscript) of the chemical symbol and the atomic number is placed at the lower. How do we represent an atom, with all of its protons, neutrons, and electrons? Identify the type of nuclear decay.
How to write an atomic symbol. Define the atomic mass unit and average atomic mass. The nuclear symbol consists of three parts:

/oxygen-chemical-element-186450996-5810f05f3df78c2c7313f35a.jpg)





![2.1/S1.2.1 Nuclear Symbol Equations [SL IB Chemistry] YouTube](https://i.ytimg.com/vi/UlQZZ_vB2DE/maxresdefault.jpg)